Question
Among the following, which one is a wrong statement?
A.
$$P{H_5}$$ and $$BiC{l_5}$$ do not exist
B.
$$p\pi - d\pi $$ bonds are present in $$S{O_2}$$
C.
$$Se{F_4}$$ and $$C{H_4}$$ have same shape
D.
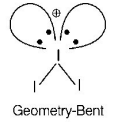
$$I_3^ + $$ has bent geometry
Answer :
$$Se{F_4}$$ and $$C{H_4}$$ have same shape
Solution :
$$P{H_5}$$ does not exist due to very less electronegativity difference between $$P$$ and $$H.$$
Hydrogen is slightly more electronegative than phosphorus, thus could not hold significantly the sharing electrons.
On the other hand, $$BiC{l_5}$$ does not exist due to inert pair effect.
On moving down the group, +5 oxidation state becomes less stable while +3 oxidation state becomes more stable.
In $$S{O_2},p\pi - d\pi $$ and $$p\pi - p\pi $$ both types of bonds are present
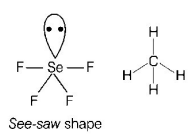
Thus, $$Se{F_4}$$ and $$C{H_4}$$ do not have same shape.
Thus, option (C) is incorrect statement.
$$P{H_5}$$ does not exist due to very less electronegativity difference between $$P$$ and $$H.$$
Hydrogen is slightly more electronegative than phosphorus, thus could not hold significantly the sharing electrons.
On the other hand, $$BiC{l_5}$$ does not exist due to inert pair effect.
On moving down the group, +5 oxidation state becomes less stable while +3 oxidation state becomes more stable.
In $$S{O_2},p\pi - d\pi $$ and $$p\pi - p\pi $$ both types of bonds are present

Thus, $$Se{F_4}$$ and $$C{H_4}$$ do not have same shape.

Thus, option (C) is incorrect statement.