Question
Among the following ions the $$p\pi - d\pi $$ overlap could be present in
A.
$$NO_2^ - $$
B.
$$NO_3^ - $$
C.
$$PO_4^{3 - }$$
D.
$$CO_3^{2 - }$$
Answer :
$$PO_4^{3 - }$$
Solution :
In $$P - O$$ bond, $$\pi - {\text{bond}}$$ is formed by the sidewise overlapping of $$d - {\text{orbital}}$$ of $$P$$ and $$p - {\text{orbital}}$$ of oxygen.
Hence, it is formed by $$p\pi $$ and $$d\pi - $$ overlapping.
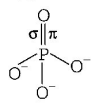
In nitrogen and carbon, no vacant $$d - {\text{orbital}}$$ is present. So, they do not form $$p\pi - d\pi $$ bond.
In $$P - O$$ bond, $$\pi - {\text{bond}}$$ is formed by the sidewise overlapping of $$d - {\text{orbital}}$$ of $$P$$ and $$p - {\text{orbital}}$$ of oxygen.
Hence, it is formed by $$p\pi $$ and $$d\pi - $$ overlapping.

In nitrogen and carbon, no vacant $$d - {\text{orbital}}$$ is present. So, they do not form $$p\pi - d\pi $$ bond.