Question
Among the following free radical bromination reactions, select those in which \[{{2}^{\circ }}\] halide is the major product
Among the following free radical bromination reactions, select those in which \[{{2}^{\circ }}\] halide is the major product
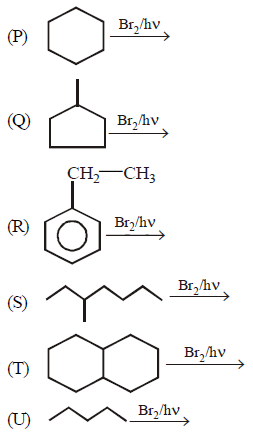
A.
$$P, Q, R, S$$
B.
$$P, R, U$$
C.
$$P, R, S, T$$
D.
$$P, Q, R, S, T$$
Answer :
$$P, R, U$$
Solution :
Bromine is more selective
∴ it will form \[{{3}^{\circ }}\] halide if there is presence of \[{{3}^{\circ }}\] hydrogen.
∴ $$Q, S, T,$$ form \[{{3}^{\circ }}\] halide as major and $$P, R, U$$ form \[{{2}^{\circ }}\] halide as major.
Bromine is more selective
∴ it will form \[{{3}^{\circ }}\] halide if there is presence of \[{{3}^{\circ }}\] hydrogen.
∴ $$Q, S, T,$$ form \[{{3}^{\circ }}\] halide as major and $$P, R, U$$ form \[{{2}^{\circ }}\] halide as major.