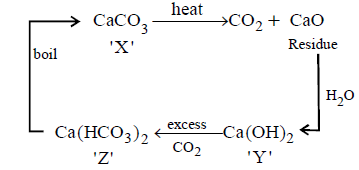Question
A solid compound $$'X'$$ on heating gives $$C{O_2}$$ gas and a residue. The residue mixed with water forms $$'Y'.$$ On passing an excess of $$C{O_2}$$ through $$'Y'$$ in water, a clear solution $$'Z',$$ is obtained. On boiling $$'Z',$$ compound $$'X'$$ is reformed. The compound $$'X'$$ is
A.
$$Ca{\left( {HC{O_3}} \right)_2}$$
B.
$$CaC{O_3}$$
C.
$$N{a_2}C{O_3}$$
D.
$${K_2}C{O_3}$$
Answer :
$$CaC{O_3}$$
Solution :

The given properties coincide with $$CaC{O_3}$$
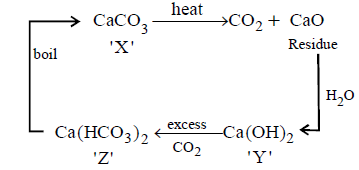

The given properties coincide with $$CaC{O_3}$$