Question
A homogeneous catalytic reaction takes place through the three alternative plots $$A,B$$ and $$C$$ shown in the given figure. Which one of the following indicates the relative ease with which the reaction can take place?
A homogeneous catalytic reaction takes place through the three alternative plots $$A,B$$ and $$C$$ shown in the given figure. Which one of the following indicates the relative ease with which the reaction can take place?
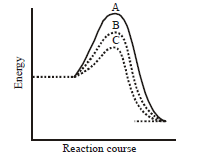
A.
$$A > B > C$$
B.
$$C > B > A$$
C.
$$A > C > B$$
D.
$$A = B = C$$
Answer :
$$C > B > A$$
Solution :
More activations energy, slow reaction rate.
More activations energy, slow reaction rate.