Question
A graph of volume of hydrogen released vs time for the reaction between zinc and dil. $$HCl$$ is given in figure. On the basis of this mark the correct option.
A graph of volume of hydrogen released vs time for the reaction between zinc and dil. $$HCl$$ is given in figure. On the basis of this mark the correct option.
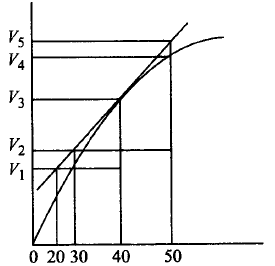
A.
Average rate upto 40 seconds is $$\frac{{{V_3} - {V_2}}}{{40}}$$
B.
Average rate upto 40 seconds is $$\frac{{{V_3} - {V_2}}}{{40 - 30}}$$
C.
Average rate upto 40 seconds is $$\frac{{{V_3}}}{{40}}$$
D.
Average rate upto 40 seconds is $$\frac{{{V_3} - {V_1}}}{{40 - 20}}$$
Answer :
Average rate upto 40 seconds is $$\frac{{{V_3}}}{{40}}$$
Solution :
Average rate of reaction upto 40 sec $$ = \frac{{{V_3} - {V_0}}}{{40 - 0}} = \frac{{{V_3}}}{{40}}$$
Average rate of reaction upto 40 sec $$ = \frac{{{V_3} - {V_0}}}{{40 - 0}} = \frac{{{V_3}}}{{40}}$$