Question
A fixed mass $$'m'$$ of a gas is subjected to transformation
of states from $$K$$ to $$L$$ to $$M$$ to $$N$$ and back to $$K$$ as shown in the figure :
A fixed mass $$'m'$$ of a gas is subjected to transformation
of states from $$K$$ to $$L$$ to $$M$$ to $$N$$ and back to $$K$$ as shown in the figure :
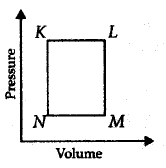
The pair of isochoric processes among the transformation of states is
A.
$$K$$ to $$L$$ and $$L$$ to $$M$$
B.
$$L$$ to $$M$$ and $$N$$ to $$K$$
C.
$$L$$ to $$M$$ and $$M$$ to $$N$$
D.
$$M$$ to $$N$$ and $$N$$ to $$K$$
Answer :
$$L$$ to $$M$$ and $$N$$ to $$K$$
Solution :
In isochoric process, volume is constant.
In isochoric process, volume is constant.