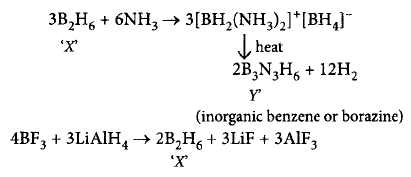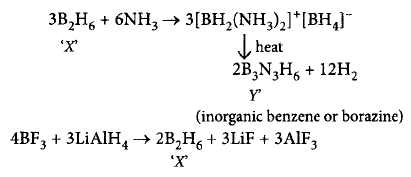Question
A compound $$X,$$ of boron reacts with $$N{H_3}$$ on heating to give another compound $$Y$$ which is called inorganic benzene. The compound $$X$$ can be prepared by treating $$B{F_3}$$ with lithium aluminium hydride. The compounds $$X$$ and $$Y$$ are represented by the formulas
A.
$${B_2}{H_6},{B_3}{N_3}{H_6}$$
B.
$${B_2}{O_3},{B_3}{N_3}{H_6}$$
C.
$$B{F_3},{B_3}{N_3}{H_6}$$
D.
$${B_3}{N_3}{H_6},{B_2}{H_6}$$
Answer :
$${B_2}{H_6},{B_3}{N_3}{H_6}$$
Solution :