Question
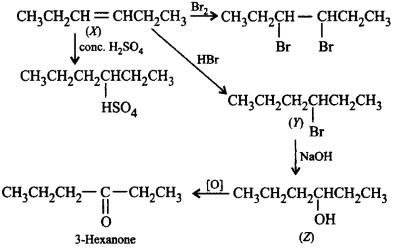
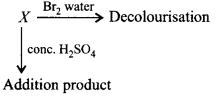
A compound $$X$$ decolourises $$B{r_2}$$ water and reacts slowly with conc. $${H_2}S{O_4}$$ to givean addition product. $$X$$ reacts with $$HBr$$ to form $$Y.$$ $$Y$$ reacts with $$NaOH$$ to form $$Z.$$ On oxidation $$Z$$ gives hexan-3-one. $$X, Y$$ and $$Z$$ in the reactions are
A compound $$X$$ decolourises $$B{r_2}$$ water and reacts slowly with conc. $${H_2}S{O_4}$$ to givean addition product. $$X$$ reacts with $$HBr$$ to form $$Y.$$ $$Y$$ reacts with $$NaOH$$ to form $$Z.$$ On oxidation $$Z$$ gives hexan-3-one. $$X, Y$$ and $$Z$$ in the reactions are
\[X\xrightarrow{HBr}Y\xrightarrow{NaOH}Z\xrightarrow{\left[ O \right]}\] \[\underset{\begin{align}
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\parallel \\
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,O \\
& \,\,\,\,\,\,\,\,\,\,\,\text{Hexan-3-one} \\
& \\
\end{align}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}-C-C{{H}_{2}}C{{H}_{3}}}}\,\]
A.
$$X = C{H_3}C{H_2}CH = CHC{H_3},$$ $$Y = C{H_3}C{H_2}CH\left( {Br} \right)CH\left( {Br} \right)C{H_2}C{H_3},$$ $$Z = C{H_3}C{H_2}C{H_3}$$
B.
$$X = C{H_3}CH = CHC{H_3},$$ $$Y = C{H_3}CH\left( {Br} \right)CH\left( {Br} \right)C{H_3},$$ $$Z = C{H_3}C{H_2}C{H_2}OH$$
C.
$$X = C{H_3}C{H_2}CH = CHC{H_2}C{H_3},$$ \[Y=C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
Br\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}C{{H}_{2}}C{{H}_{3}},\] \[Z=C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}C{{H}_{3}}\]
D.
$$X = C{H_3}C{H_2}C{H_2}CH = CHC{H_3},$$ $$Y = C{H_3}C{H_2}C{H_2}C{H_2}C{H_2}C{H_2}Br,$$ $$Z = C{H_3}C{H_2}C{H_2}C{H_2}OH$$
Answer :
$$X = C{H_3}C{H_2}CH = CHC{H_2}C{H_3},$$ \[Y=C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
Br\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}C{{H}_{2}}C{{H}_{3}},\] \[Z=C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}C{{H}_{3}}\]
Solution :