Question
A compound $$'A'$$ having the molecular formula $${C_5}{H_{12}}O,$$ on oxidation gives a compound $$'B'$$ with molecular formula $${C_5}{H_{10}}O.$$ Compound $$'B'$$ gave a 2, 4-dinitrophenylhydrazine derivative but did not answer haloform test or silver mirror test. The structure of compound $$'A'$$ is
A.
$$C{H_3} - C{H_2} - C{H_2} - C{H_2} - C{H_2}$$ $$ - OH$$
B.
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{3}}\]
C.
\[C{{H}_{3}}-C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}-C{{H}_{3}}\]
D.
\[C{{H}_{3}}-C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
C{{H}_{3}}\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}-OH\]
Answer :
\[C{{H}_{3}}-C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}-C{{H}_{3}}\]
Solution :

Since $$(B)$$ on reaction with $$2,4{\text{ - }}DNP$$ forms a derivative, it implies that $$(B)$$ has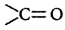 group.
group.
$$(B)$$ gives $$-ve$$ Tollens' test, hence it is not an aldehyde, but it is a ketone.
$$(B)$$ gives $$-ve$$ haloform test, thus it is not a methyl ketone.
$$(B)$$ is formed from the oxidation of $$(A),$$ thus $$(A)$$ is a $${2^ \circ }$$ alcohol, and among the given options,
$$(A)$$ is \[C{{H}_{3}}-C{{H}_{2}}\underset{\begin{smallmatrix} |\,\,\,\,\, \\ OH\,\, \end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}-C{{H}_{3}}\]
and $$(B)$$ is \[C{{H}_{3}}-C{{H}_{2}}\underset{\begin{smallmatrix} \parallel \\ O \end{smallmatrix}}{\mathop{-C-}}\,C{{H}_{2}}-C{{H}_{3}}\]

Since $$(B)$$ on reaction with $$2,4{\text{ - }}DNP$$ forms a derivative, it implies that $$(B)$$ has
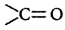 group.
group.$$(B)$$ gives $$-ve$$ Tollens' test, hence it is not an aldehyde, but it is a ketone.
$$(B)$$ gives $$-ve$$ haloform test, thus it is not a methyl ketone.
$$(B)$$ is formed from the oxidation of $$(A),$$ thus $$(A)$$ is a $${2^ \circ }$$ alcohol, and among the given options,
$$(A)$$ is \[C{{H}_{3}}-C{{H}_{2}}\underset{\begin{smallmatrix} |\,\,\,\,\, \\ OH\,\, \end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}-C{{H}_{3}}\]
and $$(B)$$ is \[C{{H}_{3}}-C{{H}_{2}}\underset{\begin{smallmatrix} \parallel \\ O \end{smallmatrix}}{\mathop{-C-}}\,C{{H}_{2}}-C{{H}_{3}}\]