Question
Which of the following figures represent the variation of particle momentum and the associated de-Broglie wavelength?
A.
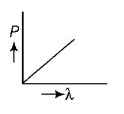

B.
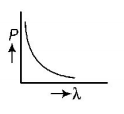

C.


D.


Answer :


Solution :
The de-Broglie wavelength is given by
$$\lambda = \frac{h}{P} \Rightarrow P\lambda = h$$
This equation is in the form of $$yx = c,$$ which is the equation of a rectangular hyperbola. Hence, the graph given in option (B) is the correct one.
The de-Broglie wavelength is given by
$$\lambda = \frac{h}{P} \Rightarrow P\lambda = h$$
This equation is in the form of $$yx = c,$$ which is the equation of a rectangular hyperbola. Hence, the graph given in option (B) is the correct one.