Question
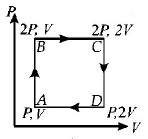
Thermodynamic processes are indicated in the following diagram
Thermodynamic processes are indicated in the following diagram
Match the following :
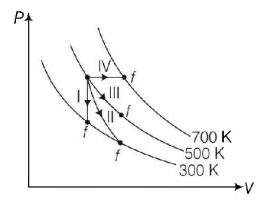
| Column-I | Column-II | ||
|---|---|---|---|
| P. | Process I | a. | Adiabatic |
| Q. | Process II | b. | Isobaric |
| R. | Process III | c. | Isochoric |
| S. | Process IV | d. | Isothermal |
A.
P → a, Q → c, R → d, S → b
B.
P → c, Q → a, R → d, S → b
C.
P → c, Q → d, R → b, S → a
D.
P → d, Q → b, R → a, S → c
Answer :
P → c, Q → a, R → d, S → b
Solution :
In isochoric process, the curve is parallel to $$y$$-axis because volume is constant. Isobaric is parallel to $$x$$-axis because pressure is constant. Along the curve, it will be isothermal because temperature is constant.
So, P → c ⇒ Q → a ⇒ R → d ⇒ S → b
In isochoric process, the curve is parallel to $$y$$-axis because volume is constant. Isobaric is parallel to $$x$$-axis because pressure is constant. Along the curve, it will be isothermal because temperature is constant.
So, P → c ⇒ Q → a ⇒ R → d ⇒ S → b