Question
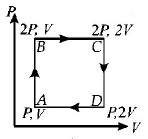
The $$PT$$ diagram for an ideal gas is shown in the figure, where $$AC$$ is an adiabatic process, find the corresponding $$PV$$ diagram.
The $$PT$$ diagram for an ideal gas is shown in the figure, where $$AC$$ is an adiabatic process, find the corresponding $$PV$$ diagram.
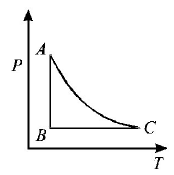
A.
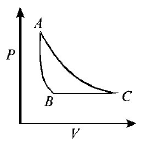

B.
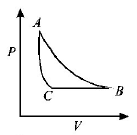

C.

D.
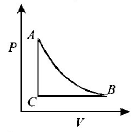

Answer :


Solution :
If we study the $$P - T$$ graph we find $$AB$$ to be a isothermal process, $$AC$$ is adiabatic process given. Also for an expansion process, the slope of adiabatic curve is more (or we can say that the area under the $$P - V$$ graph for isothermal process is more than adiabatic process for same increase in volume).
Only graph (B) fits the above criteria.
If we study the $$P - T$$ graph we find $$AB$$ to be a isothermal process, $$AC$$ is adiabatic process given. Also for an expansion process, the slope of adiabatic curve is more (or we can say that the area under the $$P - V$$ graph for isothermal process is more than adiabatic process for same increase in volume).
Only graph (B) fits the above criteria.