Question
The figure shows a plot of photocurrent versus anode potential for a photo sensitive surface for three different radiations. Which one of the following is a correct statement ?
The figure shows a plot of photocurrent versus anode potential for a photo sensitive surface for three different radiations. Which one of the following is a correct statement ?

A.
Curves $$a$$ and $$b$$ represent incident radiations of different frequencies and different intensities
B.
Curves $$a$$ and $$b$$ represent incident radiations of same frequency but of different intensities
C.
Curves $$b$$ and $$c$$ represent incident radiations of different frequencies and different intensities
D.
Curves $$b$$ and $$c$$ represent incident radiations of same frequency having same intensity
Answer :
Curves $$a$$ and $$b$$ represent incident radiations of same frequency but of different intensities
Solution :
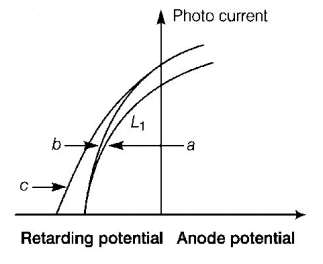
Since in the graph retarding potential is same in graph (a) and (b) and photo current is different so, for curves they have same frequency but different intensity of light.

Since in the graph retarding potential is same in graph (a) and (b) and photo current is different so, for curves they have same frequency but different intensity of light.