Question
Suppose $$0.5\,mole$$ of an ideal gas undergoes an isothermal expansion as energy is added to its heat $$Q.$$
Suppose $$0.5\,mole$$ of an ideal gas undergoes an isothermal expansion as energy is added to its heat $$Q.$$
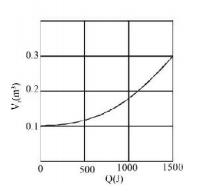
Graph shows the final volume $${V_f}$$ versus $$Q.$$ The temperature of the gas is (use $$\ln 9 = 2$$ and $$R = \frac{{25}}{3}\,J/mol - K$$ )
A.
$$360\,K$$
B.
$$293\,K$$
C.
$$386\,K$$
D.
$$412\,K$$
Answer :
$$360\,K$$
Solution :
$$\eqalign{ & Q = W = nRT\ln \frac{{{V_f}}}{{{V_i}}} \cr & T = \frac{Q}{{nR\ln \left( {\frac{{{V_f}}}{{{V_i}}}} \right)}} = \frac{{1500}}{{\frac{{0.5 \times 25}}{{3 \times \ln 3}}}} \cr & \Rightarrow T = \frac{{1500}}{{\frac{{0.5 \times 25}}{{3 \times 1}}}} = 360\,K \cr} $$
$$\eqalign{ & Q = W = nRT\ln \frac{{{V_f}}}{{{V_i}}} \cr & T = \frac{Q}{{nR\ln \left( {\frac{{{V_f}}}{{{V_i}}}} \right)}} = \frac{{1500}}{{\frac{{0.5 \times 25}}{{3 \times \ln 3}}}} \cr & \Rightarrow T = \frac{{1500}}{{\frac{{0.5 \times 25}}{{3 \times 1}}}} = 360\,K \cr} $$