Question
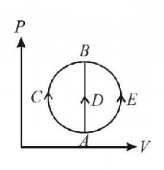
One mole of an ideal gas is taken from state $$A$$ to state $$B$$ by three different processes, (i) $$ACB$$ (ii) $$ADB$$ (iii) $$AEB$$ as shown in the $$P-V$$ diagram. The heat absorbed by the gas is -
One mole of an ideal gas is taken from state $$A$$ to state $$B$$ by three different processes, (i) $$ACB$$ (ii) $$ADB$$ (iii) $$AEB$$ as shown in the $$P-V$$ diagram. The heat absorbed by the gas is -
A.
greater in process (ii) than in (i)
B.
the least in process (ii)
C.
the same in (i) and (iii)
D.
less in (iii) than in (ii)
Answer :
less in (iii) than in (ii)
Solution :
Heat absorbed by gas in three processes is given by
$$\eqalign{ & {Q_{ACB}} = \Delta U + {W_{ACB}} \cr & {Q_{ADB}} = \Delta U \cr & {Q_{AEB}} = \Delta U + {W_{AEB}} \cr} $$
The change in internal energy in all the three cases is same and $${W_{ACB}}$$ is $$ + ve,\,{W_{AEB}}$$ is $$-ve.$$
Hence $${Q_{ACB}} > {Q_{ADB}} > {Q_{AEB}}$$
Heat absorbed by gas in three processes is given by
$$\eqalign{ & {Q_{ACB}} = \Delta U + {W_{ACB}} \cr & {Q_{ADB}} = \Delta U \cr & {Q_{AEB}} = \Delta U + {W_{AEB}} \cr} $$
The change in internal energy in all the three cases is same and $${W_{ACB}}$$ is $$ + ve,\,{W_{AEB}}$$ is $$-ve.$$
Hence $${Q_{ACB}} > {Q_{ADB}} > {Q_{AEB}}$$