Question
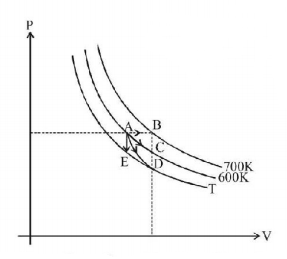
For an ideal gas four processes are marked as 1,2,3 and 4 on $$P-V$$ diagram as shown in figure. The amount of heat supplied to the gas in the process 1, 2, 3 and 4 are $${Q_1},{Q_2},{Q_3}$$ and $${Q_4}$$ respectively, then correct order of heat supplied to the gas is - [$$AB$$ is process-1, $$AC$$ is process-2, $$AD$$ is adiabatic process-3 and $$AE$$ is process-4]
For an ideal gas four processes are marked as 1,2,3 and 4 on $$P-V$$ diagram as shown in figure. The amount of heat supplied to the gas in the process 1, 2, 3 and 4 are $${Q_1},{Q_2},{Q_3}$$ and $${Q_4}$$ respectively, then correct order of heat supplied to the gas is - [$$AB$$ is process-1, $$AC$$ is process-2, $$AD$$ is adiabatic process-3 and $$AE$$ is process-4]
A.
$${Q_1} > {Q_2} > {Q_3} > {Q_4}$$
B.
$${Q_1} > {Q_2} > {Q_4} > {Q_3}$$
C.
$${Q_1} > {Q_4} > {Q_2} > {Q_3}$$
D.
$${Q_1} < {Q_2} < {Q_3} < {Q_4}$$
Answer :
$${Q_1} > {Q_2} > {Q_4} > {Q_3}$$
Solution :
In process-1 heat supplied $$=$$ area under $$AB$$ curve + $$n \times {c_v} \times 100\,\left( {{\text{isobaric process}}} \right)$$
In process-2 heat supplied $$=$$ area under $$AC$$ curve (isothermal process)
In process-3 heat supplied $$= 0$$ (adiabatic process)
In process-4 heat supplied $$ = n \times {c_v}\,\left( {T - 600} \right)\,\left( {{\text{isobaric process}}} \right)$$
In process-1 heat supplied $$=$$ area under $$AB$$ curve + $$n \times {c_v} \times 100\,\left( {{\text{isobaric process}}} \right)$$
In process-2 heat supplied $$=$$ area under $$AC$$ curve (isothermal process)
In process-3 heat supplied $$= 0$$ (adiabatic process)
In process-4 heat supplied $$ = n \times {c_v}\,\left( {T - 600} \right)\,\left( {{\text{isobaric process}}} \right)$$