Question
An ideal gas is initially at $${P_1},{V_1}$$ is expanded to $${P_2},{V_2}$$ and then compressed adiabatically to the same volume $${V_1}$$ and pressure $${P_3}.$$ If $$W$$ is the net work done by the gas in complete process which of the following is true?
A.
$$W > 0;{P_3} > {P_1}$$
B.
$$W < 0;{P_3} > {P_1}$$
C.
$$W > 0;{P_3} < {P_1}$$
D.
$$W < 0;{P_3} < {P_1}$$
Answer :
$$W < 0;{P_3} > {P_1}$$
Solution :
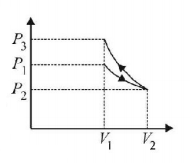
In the first process $$W$$ is $$+ ve$$ as $$\Delta V$$ is positive, in the second process $$W$$ is $$-ve$$ as $$\Delta V$$ is $$-ve$$ and area under the curve of second process is more
$$\therefore {\text{Net}}\,{\text{Work}}\, < 0\,{\text{and}}\,{\text{also}}\,{P_3} > {P_1}.$$

In the first process $$W$$ is $$+ ve$$ as $$\Delta V$$ is positive, in the second process $$W$$ is $$-ve$$ as $$\Delta V$$ is $$-ve$$ and area under the curve of second process is more
$$\therefore {\text{Net}}\,{\text{Work}}\, < 0\,{\text{and}}\,{\text{also}}\,{P_3} > {P_1}.$$