Question
$$Xe{O_4}$$ molecule is tetrahedral having :
A.
Two $$p\pi - d\pi $$ bonds
B.
One $$p\pi - d\pi $$ bonds
C.
Four $$p\pi - d\pi $$ bonds
D.
Three $$p\pi - d\pi $$ bonds
Answer :
Four $$p\pi - d\pi $$ bonds
Solution :
Xenon undergo $$s{p^3}$$ hybridization.

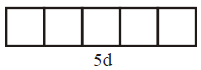 ( ground state )
( ground state )

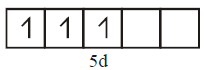 ( third excited state )
( third excited state )
In the fourth excited state xenon atom, has 8 unpaired electrons
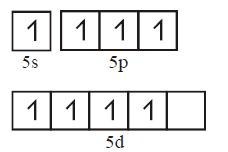
One $$s$$ and three $$p$$ orbital undergo $$s{p^3}$$ hybridization. Four $$s{p^3}$$ hybrid orbitals form four $$\sigma $$ bonds with oxygen atoms. They are $$\sigma s{p^3} - p.$$ Four $$p\pi - d\pi $$ bonds are also formed with oxygen atoms by the unpaired electrons.
Xenon undergo $$s{p^3}$$ hybridization.

 ( ground state )
( ground state )
 ( third excited state )
( third excited state )In the fourth excited state xenon atom, has 8 unpaired electrons

One $$s$$ and three $$p$$ orbital undergo $$s{p^3}$$ hybridization. Four $$s{p^3}$$ hybrid orbitals form four $$\sigma $$ bonds with oxygen atoms. They are $$\sigma s{p^3} - p.$$ Four $$p\pi - d\pi $$ bonds are also formed with oxygen atoms by the unpaired electrons.