Question
Which of the following statements is not valid for oxoacids of phosphorus?
A.
Orthophosphoric acid is used in the manufacture of triple superphosphate
B.
Hypophosphorous acid is a diprotic acid
C.
All oxoacids contain tetrahedral four coordinated phosphorus
D.
All oxoacids contain at least one $$P =O$$ unit and one $$P—OH$$ group
Answer :
Hypophosphorous acid is a diprotic acid
Solution :
Hypophosphorous acid, $${H_3}P{O_2},$$ has the following structure.
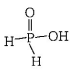
As it contains only one replaceable $$H$$-atom ( that is attached with $$O,$$ not with $$P$$ directly ) so it is a monoprotic acid.
All other given statements are true.
Hypophosphorous acid, $${H_3}P{O_2},$$ has the following structure.

As it contains only one replaceable $$H$$-atom ( that is attached with $$O,$$ not with $$P$$ directly ) so it is a monoprotic acid.
All other given statements are true.