Question
Which of the following statements is not true about the stability of carbanions?
A.
Stability of carbanions increases with increase in $$s$$ - character of orbital.
B.
The electron withdrawing groups like $$ - N{O_2},-CN,$$ 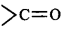 increases the stability of carbanions.
increases the stability of carbanions.
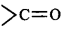 increases the stability of carbanions.
increases the stability of carbanions. C.
Order of stability of carbanions is $${3^ \circ } > {2^ \circ } > {1^ \circ }.$$
D.
The negatively charged carbon is $$s{p^3}$$ hybridised and pyramidal.
Answer :
Order of stability of carbanions is $${3^ \circ } > {2^ \circ } > {1^ \circ }.$$
Solution :
Order of stability of carbanions is $${1^ \circ } > {2^ \circ } > {3^ \circ }.$$
Order of stability of carbanions is $${1^ \circ } > {2^ \circ } > {3^ \circ }.$$