Question
Which of the following order is wrong?
A.
$$N{H_3} < P{H_3} < As{H_3} - $$ Acidic
B.
$$Li < Be < B < C - $$ 1st Ionisation potential
C.
$$A{l_2}{O_3} < MgO < N{a_2}O < {K_2}O - $$ Basic
D.
$$L{i^ + } < N{a^ + } < {K^ + } < C{s^ + } - $$ Ionic radius
Answer :
$$Li < Be < B < C - $$ 1st Ionisation potential
Solution :
$$Li, Be, B$$ and $$C$$ are present in IInd period. In a period from left to right ionisation potential increases.
\[\xrightarrow[Li\,\,\,Be\,\,\,\,B\,\,\,\,C]{\text{Ionisation}\,\,\text{potention}\,\,\text{increase}}\]
* But in case of $$Be$$ and $$B, Be$$ has higher ionisation potential than $$B$$ due to stable configuration of $$Be.$$
$${}_4Be = 1{s^2},2{s^2}$$
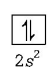 Stable configuration ( due to fully- filled orbital )
Stable configuration ( due to fully- filled orbital )
$${}_5B = 1{s^2},2{s^2}2{p^1}$$
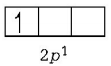 Unstable configuration
Unstable configuration
So, the correct order of ionisation potential of given elements is
$$Li < B < Be < C$$
$$Li, Be, B$$ and $$C$$ are present in IInd period. In a period from left to right ionisation potential increases.
\[\xrightarrow[Li\,\,\,Be\,\,\,\,B\,\,\,\,C]{\text{Ionisation}\,\,\text{potention}\,\,\text{increase}}\]
* But in case of $$Be$$ and $$B, Be$$ has higher ionisation potential than $$B$$ due to stable configuration of $$Be.$$
$${}_4Be = 1{s^2},2{s^2}$$
 Stable configuration ( due to fully- filled orbital )
Stable configuration ( due to fully- filled orbital ) $${}_5B = 1{s^2},2{s^2}2{p^1}$$
 Unstable configuration
Unstable configuration So, the correct order of ionisation potential of given elements is
$$Li < B < Be < C$$