Question
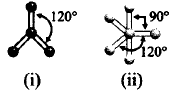
Which molecule is depicted by the given ball and stick models?
Which molecule is depicted by the given ball and stick models?
A.
$$\left( {\text{i}} \right)BeC{l_2},\left( {{\text{ii}}} \right)C{H_4}$$
B.
$$\left( {\text{i}} \right)B{F_3},\left( {{\text{ii}}} \right)PC{l_5}$$
C.
$$\left( {\text{i}} \right)B{F_4},\left( {{\text{ii}}} \right)C{H_4}$$
D.
$$\left( {\text{i}} \right)BeC{l_2},\left( {{\text{ii}}} \right)PC{l_5}$$
Answer :
$$\left( {\text{i}} \right)B{F_3},\left( {{\text{ii}}} \right)PC{l_5}$$
Solution :
$$B{F_3} - $$ Trigonal planar with bond angle $${120^ \circ }.$$
$$PC{l_5} - $$ Trigonal bipyramidal with bond angles $${120^ \circ }$$ and $${90^ \circ }.$$
$$B{F_3} - $$ Trigonal planar with bond angle $${120^ \circ }.$$
$$PC{l_5} - $$ Trigonal bipyramidal with bond angles $${120^ \circ }$$ and $${90^ \circ }.$$