Question
When a system is taken from state $$i$$ to state $$f$$ along the path $$iaf,$$ it is found that $$Q = 50\,cal$$ and $$W = 20\,cal.$$ Along the path $$ibf\,Q = 36\,cal.$$ $$W$$ along the path $$ibf$$ is
When a system is taken from state $$i$$ to state $$f$$ along the path $$iaf,$$ it is found that $$Q = 50\,cal$$ and $$W = 20\,cal.$$ Along the path $$ibf\,Q = 36\,cal.$$ $$W$$ along the path $$ibf$$ is

A.
$$14\,cal$$
B.
$$6\,cal$$
C.
$$16\,cal$$
D.
$$66\,cal$$
Answer :
$$6\,cal$$
Solution :
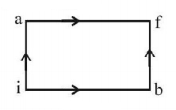
For path $$iaf,$$
$$Q = 50\,cal$$
$$W = 20\,cal$$
By first law of thermodynamics,
$$\Delta U = Q - W = 50 - 20 = 30\,cal.$$
For path $$ibf$$
$$\eqalign{ & Q' = 36\,cal \cr & W' = ? \cr & {\text{or,}}\,W' = Q' - \Delta U' \cr} $$
Since, the change in internal energy does not depend on the path, therefore
$$\eqalign{ & \Delta U' = 30\,cal \cr & \therefore W' = Q' - \Delta U' = 36 - 30 = 6\,cal. \cr} $$

For path $$iaf,$$
$$Q = 50\,cal$$
$$W = 20\,cal$$
By first law of thermodynamics,
$$\Delta U = Q - W = 50 - 20 = 30\,cal.$$
For path $$ibf$$
$$\eqalign{ & Q' = 36\,cal \cr & W' = ? \cr & {\text{or,}}\,W' = Q' - \Delta U' \cr} $$
Since, the change in internal energy does not depend on the path, therefore
$$\eqalign{ & \Delta U' = 30\,cal \cr & \therefore W' = Q' - \Delta U' = 36 - 30 = 6\,cal. \cr} $$
