Question
Toluene is nitrated and the resulting product is reduced with tin and hydrochloric acid. The product so obtained is diazotised and then heated wth cuprous bromide. The reaction mixture so formed contans
A.
mixture of $$o - $$ and $$p - $$ bromotoluenes
B.
mixture of $$o - $$ and $$p - $$ dibromobenzenes
C.
mixture of $$o - $$ and $$p - $$ bromoanilines
D.
mixture of $$o - $$ and $$m - $$ bromotoluenes
Answer :
mixture of $$o - $$ and $$p - $$ bromotoluenes
Solution :
NOTE : Toluene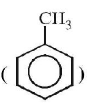 contains $$ - C{H_3}$$ group which
contains $$ - C{H_3}$$ group which
is $$o - ,p - $$ directing group so on nitration of toluene the $$ - N{O_2}$$ group will occupy $$o - ,p - $$ poditions.
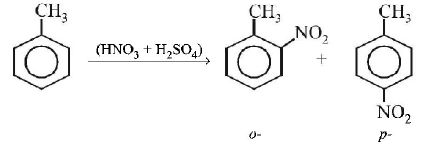
on reduction with $$Sn/HCl$$ they will form corresponding anilines in which $$ - N{O_2}$$ group changes to $$ - N{H_2}.$$ The
mixture now contains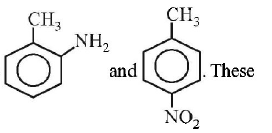
anilines when diazotized and then treated with $$CuBr$$ forms $$o - ,p - $$ bromotoluenes.
NOTE : Toluene
 contains $$ - C{H_3}$$ group which
contains $$ - C{H_3}$$ group whichis $$o - ,p - $$ directing group so on nitration of toluene the $$ - N{O_2}$$ group will occupy $$o - ,p - $$ poditions.

on reduction with $$Sn/HCl$$ they will form corresponding anilines in which $$ - N{O_2}$$ group changes to $$ - N{H_2}.$$ The
mixture now contains

anilines when diazotized and then treated with $$CuBr$$ forms $$o - ,p - $$ bromotoluenes.