Question
The ground state electronic configuration of $$S$$ is $$3{s^2}3{p^4}.$$ How does it form the compound $$S{F_6}?$$
A.
Due to octahedral shape of $$S$$ atoms.
B.
Due to presence of vacant 3 $$d$$ - orbitals which provide 6 unpaired electrons in excited state.
C.
Due to $$s{p^3}$$ hybridisation of $$S$$ atom which provides 6 electrons to 6 $$F$$ atoms.
D.
Due to presence of 3 $$sigma$$ and 3 $$pi$$ bonds between $$S$$ and $$F.$$
Answer :
Due to presence of vacant 3 $$d$$ - orbitals which provide 6 unpaired electrons in excited state.
Solution :
$$S$$ can go into excited state with 6 unpaired electrons due to presence of vacant 3 $$d$$ - orbitals, which are overlapped by six $$F$$ electrons.
Ground state :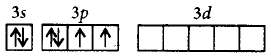
Excited state :
$$S$$ can go into excited state with 6 unpaired electrons due to presence of vacant 3 $$d$$ - orbitals, which are overlapped by six $$F$$ electrons.
Ground state :

Excited state :
