Question
The correct statement regarding the basicity of arylamines is
A.
Arylamines are generally more basic than alkylamines because the nitrogen lone-pair electrons are not delocalized by interaction with the aromatic ring $$\pi $$ - electron system
B.
Arylamines are generally more basic than alkylamines because of aryl group
C.
Arylamines are generally more basic than alkylamines, because the nitrogen atom in arylamines is $$sp$$ - hybridized
D.
Arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring $$\pi $$ - electron system
Answer :
Arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring $$\pi $$ - electron system
Solution :
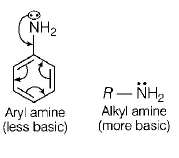
Due to delocalisation of lone pair of electrons of $$N$$ - atom to the benzene ring, it losses its basicity and becomes less basic than alkyl amine.
On the other hand, alkyl amine has free lone pair of electron as well as $$ + I$$ - effect of alkyl group increases electron density on $$N$$ - atom enhancing its basic nature.

Due to delocalisation of lone pair of electrons of $$N$$ - atom to the benzene ring, it losses its basicity and becomes less basic than alkyl amine.
On the other hand, alkyl amine has free lone pair of electron as well as $$ + I$$ - effect of alkyl group increases electron density on $$N$$ - atom enhancing its basic nature.