Question
The $$BC{l_3}$$ is a planar molecule whereas $$NC{l_3}$$ is pyramidal, because
A.
$$B - Cl$$ bond is more polar than $$N - Cl$$ bond
B.
$$N - Cl$$ bond is more covalent than $$B - Cl$$ bond
C.
nitrogen atom is smaller than boron atoms
D.
$$BC{l_3}$$ has no lone pair but $$NC{l_3}$$ has a lone pair of electrons
Answer :
$$BC{l_3}$$ has no lone pair but $$NC{l_3}$$ has a lone pair of electrons
Solution :
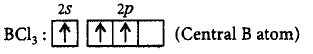
No lone pair of electrons is available in $$BC{l_3}.$$
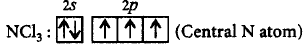
One lone pair of electrons is available on $$N$$ atom, it occupies a corner in the tetrahedral arrangement. Therefore, $$NC{l_3}$$ appears pyramidal in shape.
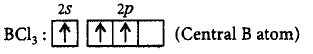
No lone pair of electrons is available in $$BC{l_3}.$$
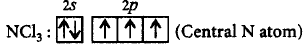
One lone pair of electrons is available on $$N$$ atom, it occupies a corner in the tetrahedral arrangement. Therefore, $$NC{l_3}$$ appears pyramidal in shape.