Question
The aqueous solution containing which one of the following ions will be colourless?
The aqueous solution containing which one of the following ions will be colourless?
$$\left( {{\text{At}}{\text{. no}}{\text{.}}\,Sc = 21,Fe = 26,} \right.$$ $$\left. {Ti = 22,Mn = 25} \right)$$
A.
$$S{c^{3 + }}$$
B.
$$F{e^{2 + }}$$
C.
$$T{i^{3 + }}$$
D.
$$M{n^{2 + }}$$
Answer :
$$S{c^{3 + }}$$
Solution :
$${}_{21}Sc = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^1},4{s^2}$$
$$So,\,\,S{c^{3 + }} = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}$$
( It is colourless due to the absence of unpaired electrons in $$d$$ sub-shell )
$${}_{26}Fe = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^6},4{s^2}$$
$$F{e^{2 + }} = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^6}$$
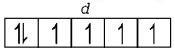
( It is coloured due to the presence of four unpaired electrons in $$d$$ sub-shell )
$${}_{22}Ti = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^2},4{s^2}$$
$$T{i^{3 + }} = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^1}$$
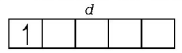
( It is coloured due to the presence of an unpaired electron in $$d$$ sub-shell )
$${}_{25}Mn = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^5},4{s^2}$$
$$M{n^{2 + }} = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^5}$$
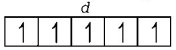
( It is coloured due to the presence of five unpaired electrons in $$d$$ sub-shell )
$${}_{21}Sc = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^1},4{s^2}$$
$$So,\,\,S{c^{3 + }} = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}$$
( It is colourless due to the absence of unpaired electrons in $$d$$ sub-shell )
$${}_{26}Fe = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^6},4{s^2}$$
$$F{e^{2 + }} = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^6}$$

( It is coloured due to the presence of four unpaired electrons in $$d$$ sub-shell )
$${}_{22}Ti = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^2},4{s^2}$$
$$T{i^{3 + }} = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^1}$$

( It is coloured due to the presence of an unpaired electron in $$d$$ sub-shell )
$${}_{25}Mn = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^5},4{s^2}$$
$$M{n^{2 + }} = 1{s^2},2{s^2}2{p^6},3{s^2}3{p^6}3{d^5}$$

( It is coloured due to the presence of five unpaired electrons in $$d$$ sub-shell )