Question
$$P - V$$ plots for two gases during adiabatic processes are shown in the figure. Plots 1 and 2 should correspond respectively to
$$P - V$$ plots for two gases during adiabatic processes are shown in the figure. Plots 1 and 2 should correspond respectively to
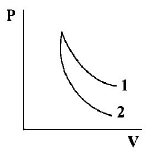
A.
$$He\,\,{\text{and }}{O_2}$$
B.
$${O_2}\,\,{\text{and }}He$$
C.
$$He\,\,{\text{and }}Ar$$
D.
$${O_2}\,\,{\text{and }}{N_2}$$
Answer :
$${O_2}\,\,{\text{and }}He$$
Solution :
For adiabatic process $$P{V^\gamma } = {\text{constant}}$$
Also for monoatomic gas $$\gamma = \frac{{{C_P}}}{{{C_V}}} = 1.67$$
for diatomic gas $$\gamma = 1.4$$
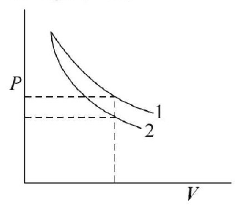
Since, $${\gamma _{{\text{diatomic}}}} < {\gamma _{{\text{mono atomic}}}}$$
$$\therefore \,\,{P_{{\text{diatomic}}}} > {P_{{\text{mono atomic}}}}$$
⇒ Graph 1 is for diatomic and graph 2 is for mono atomic.
For adiabatic process $$P{V^\gamma } = {\text{constant}}$$
Also for monoatomic gas $$\gamma = \frac{{{C_P}}}{{{C_V}}} = 1.67$$
for diatomic gas $$\gamma = 1.4$$

Since, $${\gamma _{{\text{diatomic}}}} < {\gamma _{{\text{mono atomic}}}}$$
$$\therefore \,\,{P_{{\text{diatomic}}}} > {P_{{\text{mono atomic}}}}$$
⇒ Graph 1 is for diatomic and graph 2 is for mono atomic.
