Question
Match the interhalogen compounds of Column I with the geometry in Column II and assign the correct code.
Match the interhalogen compounds of Column I with the geometry in Column II and assign the correct code.
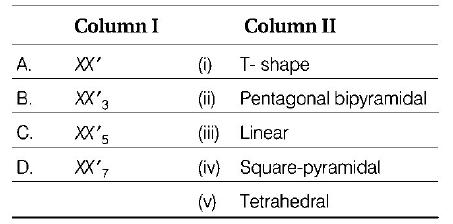
Code
$$\eqalign{
& \,\,\,\,\,\,\,\,\,\,\,\,\,{\text{A}}\,\,\,\,\,\,\,\,\,{\text{B}}\,\,\,\,\,\,\,\,\,\,{\text{C}}\,\,\,\,\,\,{\text{D}} \cr
& \left( {\text{a}} \right)\,\,\left( {{\text{iii}}} \right)\,\,\,\,\left( {{\text{iv}}} \right)\,\,\,\,\left( {\text{i}} \right)\,\,\,\,\left( {{\text{ii}}} \right) \cr
& \left( {\text{b}} \right)\,\,\left( {{\text{iii}}} \right)\,\,\,\,\,\left( {\text{i}} \right)\,\,\,\,\left( {{\text{iv}}} \right)\,\,\,\left( {{\text{ii}}} \right) \cr
& \left( {\text{c}} \right)\,\,\,\left( {\text{v}} \right)\,\,\,\,\,\left( {{\text{iv}}} \right)\,\,\,\left( {{\text{iii}}} \right)\,\,\left( {{\text{ii}}} \right) \cr
& \left( {\text{d}} \right)\,\,\left( {{\text{iv}}} \right)\,\,\,\,\left( {{\text{iii}}} \right)\,\,\,\left( {{\text{ii}}} \right)\,\,\,\,\left( {\text{i}} \right) \cr} $$
A.
(a)
B.
(b)
C.
(c)
D.
(d)
Answer :
(b)
Solution :
Two different halogens may react to form interhalogen compounds as
$$\eqalign{ & XX'\left( {ClF,BrF,BrCl,IF,ICl} \right)\,\,\,\,\,{\text{Linear}} \cr & XX{'_3}\left( {Cl{F_3},Br{F_3},I{F_3},IC{l_3}} \right)\,\,\,\,\,\,\,\,\,\,\,{\text{Bent }}T{\text{ - shaped}} \cr & XX{'_5}\left( {Cl{F_5},BrC{l_5},I{F_5}} \right)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{Square - pyramidal}} \cr & XX{'_7}\left( {I{F_7}} \right)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{Pentagonal bipyramidal}} \cr} $$
Two different halogens may react to form interhalogen compounds as
$$\eqalign{ & XX'\left( {ClF,BrF,BrCl,IF,ICl} \right)\,\,\,\,\,{\text{Linear}} \cr & XX{'_3}\left( {Cl{F_3},Br{F_3},I{F_3},IC{l_3}} \right)\,\,\,\,\,\,\,\,\,\,\,{\text{Bent }}T{\text{ - shaped}} \cr & XX{'_5}\left( {Cl{F_5},BrC{l_5},I{F_5}} \right)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{Square - pyramidal}} \cr & XX{'_7}\left( {I{F_7}} \right)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{Pentagonal bipyramidal}} \cr} $$