Question
Match the column I with column II and mark the appropriate choice.
Match the column I with column II and mark the appropriate choice.
| Column I | Column II | ||
|---|---|---|---|
| a. | $${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ | 1. | $$0\,B.M.$$ |
| b. | $${\left[ {Co{F_6}} \right]^{3 - }}$$ | 2. | $$5.92\,B.M.$$ |
| c. | $${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$ | 3. | $$4.89\,B.M.$$ |
| d. | $${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$ | 4. | $$1.732\,B.M.$$ |
A.
a - 2, b - 3, c - 4, d - 1
B.
a - 3, b - 2, c - 1, d - 4
C.
a - 1, b - 3, c - 4, d - 2
D.
a - 4, b - 3, c - 2, d - 1
Answer :
a - 4, b - 3, c - 2, d - 1
Solution :
$$F{e^{3 + }}:3{d^5},$$ since $$C{N^ - }$$ is a strong field ligand, pairing will take place.
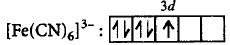 $$\left[ {\because n = 1} \right]$$
$$\left[ {\because n = 1} \right]$$
$$\eqalign{ & \mu = \sqrt {n\left( {n + 2} \right)} \cr & \,\,\,\, = \sqrt {1\left( {1 + 2} \right)} \cr & \,\,\,\, = \sqrt 3 \cr & \,\,\,\, = 1.732\,\,B.M. \cr} $$
In $${\left[ {Co{F_6}} \right]^{3 - }},$$ oxidation state of $$Co = + 3$$
$$C{o^{3 + }}:3{d^6},$$ since $${F^ - }$$ is a weak field ligand, pairing will not occur.
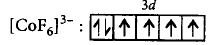 $$\left[ {\because n = 4} \right]$$
$$\left[ {\because n = 4} \right]$$
$$\eqalign{ & \mu = \sqrt {4\left( {4 + 2} \right)} \cr & \,\,\,\,\, = \sqrt {24} \cr & \,\,\,\,\, = 4.89\,B.M. \cr} $$
In $${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }},$$ oxidation state of $$Fe = + 3$$
$$F{e^{3 + }}:3{d^5},$$ since $${H_2}O$$ is a weak field ligand, pairing will not take place.
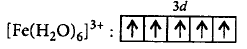 $$\left[ {\because n = 5} \right]$$
$$\left[ {\because n = 5} \right]$$
$$\eqalign{ & \mu = \sqrt {5\left( {5 + 2} \right)} \cr & \,\,\,\,\, = \sqrt {35} \cr & \,\,\,\,\, = 5.92\,B.M. \cr} $$
In $${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }},$$ oxidation state of $$Co=+3.$$
$$C{o^{3 + }}:3{d^6},$$ since $$N{H_3}$$ is a strong field ligand, pairing will take place.
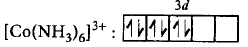 $$\left[ {\because n = 0} \right]$$
$$\left[ {\because n = 0} \right]$$
$$\mu = \sqrt {0\left( {0 + 2} \right)} = 0$$
$$F{e^{3 + }}:3{d^5},$$ since $$C{N^ - }$$ is a strong field ligand, pairing will take place.
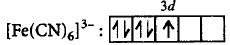 $$\left[ {\because n = 1} \right]$$
$$\left[ {\because n = 1} \right]$$$$\eqalign{ & \mu = \sqrt {n\left( {n + 2} \right)} \cr & \,\,\,\, = \sqrt {1\left( {1 + 2} \right)} \cr & \,\,\,\, = \sqrt 3 \cr & \,\,\,\, = 1.732\,\,B.M. \cr} $$
In $${\left[ {Co{F_6}} \right]^{3 - }},$$ oxidation state of $$Co = + 3$$
$$C{o^{3 + }}:3{d^6},$$ since $${F^ - }$$ is a weak field ligand, pairing will not occur.
 $$\left[ {\because n = 4} \right]$$
$$\left[ {\because n = 4} \right]$$$$\eqalign{ & \mu = \sqrt {4\left( {4 + 2} \right)} \cr & \,\,\,\,\, = \sqrt {24} \cr & \,\,\,\,\, = 4.89\,B.M. \cr} $$
In $${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }},$$ oxidation state of $$Fe = + 3$$
$$F{e^{3 + }}:3{d^5},$$ since $${H_2}O$$ is a weak field ligand, pairing will not take place.
 $$\left[ {\because n = 5} \right]$$
$$\left[ {\because n = 5} \right]$$$$\eqalign{ & \mu = \sqrt {5\left( {5 + 2} \right)} \cr & \,\,\,\,\, = \sqrt {35} \cr & \,\,\,\,\, = 5.92\,B.M. \cr} $$
In $${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }},$$ oxidation state of $$Co=+3.$$
$$C{o^{3 + }}:3{d^6},$$ since $$N{H_3}$$ is a strong field ligand, pairing will take place.
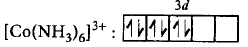 $$\left[ {\because n = 0} \right]$$
$$\left[ {\because n = 0} \right]$$$$\mu = \sqrt {0\left( {0 + 2} \right)} = 0$$