Question
Match the column I with column II and mark the appropriate choice.
Match the column I with column II and mark the appropriate choice.
| Column I | Column II | ||
|---|---|---|---|
| a. | $${C_2}{H_2}$$ | 1. | $$s{p^3}{d^2}$$ hybridisation |
| b. | $$S{F_6}$$ | 2. | $$s{p^3}{d^3}$$ hybridisation |
| c. | $$S{O_2}$$ | 3. | $$sp$$ hybridisation |
| d. | $$I{F_7}$$ | 4. | $$s{p^2}$$ hybridisation |
A.
a - 1, b - 3, c - 2, d - 4
B.
a - 3, b - 1, c - 4, d - 2
C.
a - 2, b - 3, c - 1, d - 2
D.
a - 4, b - 1, c - 3, d - 2
Answer :
a - 3, b - 1, c - 4, d - 2
Solution :
In $${C_2}{H_2},C$$ undergoes, $$sp$$ hybridisation.
Ground state :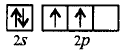
Excited state :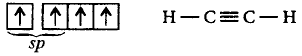
In $$S{F_6},S$$ undergoes $$s{p^3}{d^2}$$ hybridisation.
Ground state :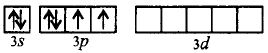
Excited state :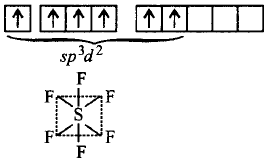
In $$S{O_2},S$$ undergoes $$s{p^2}$$ hybridisation.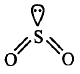
In $$I{F_7},I$$ undergoes $$s{p^3}{d^3}$$ hybridisation.
Ground state :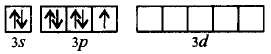
Excited state :
In $${C_2}{H_2},C$$ undergoes, $$sp$$ hybridisation.
Ground state :

Excited state :
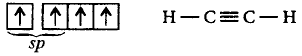
In $$S{F_6},S$$ undergoes $$s{p^3}{d^2}$$ hybridisation.
Ground state :

Excited state :

In $$S{O_2},S$$ undergoes $$s{p^2}$$ hybridisation.

In $$I{F_7},I$$ undergoes $$s{p^3}{d^3}$$ hybridisation.
Ground state :

Excited state :
