Question
In which of the following pairs, both the species are not isostructural?
A.
$$SiC{l_4},PCl_4^ + $$
B.
$${\text{Diamond, carbide}}$$
C.
$$N{H_3},P{H_3}$$
D.
$$Xe{F_4},Xe{O_4}$$
Answer :
$$Xe{F_4},Xe{O_4}$$
Solution :
(i) Structure of $$SiC{l_4}$$
.PNG)
Structure of $$PCl_4^ + $$
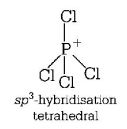
(ii) Diamond and silicon carbide $$(SiC),$$ both are isostructural because their central atom is $$s{p^3}$$ hybridised and both have tetrahedral arrangement.
(iii) Structure of $$N{H_3}$$
.PNG)
Structure of $$P{H_3}$$
.PNG)
Both $$N{H_3}$$ and $$P{H_3}$$ have $$s{p^3}$$ geometry.
(iv) $$Xe{F_4}$$ has $$s{p^3}{d^2}$$ hybridisation while $$Xe{O_4}$$ has $$s{p^3}$$ hybridisation.
Structure of $$Xe{F_4}$$
.PNG)
Structure of $$Xe{O_4}$$
.PNG)
Hence, $$Xe{F_4}$$ and $$Xe{O_4}$$ are not isostructural.
(i) Structure of $$SiC{l_4}$$
.PNG)
Structure of $$PCl_4^ + $$

(ii) Diamond and silicon carbide $$(SiC),$$ both are isostructural because their central atom is $$s{p^3}$$ hybridised and both have tetrahedral arrangement.
(iii) Structure of $$N{H_3}$$
.PNG)
Structure of $$P{H_3}$$
.PNG)
Both $$N{H_3}$$ and $$P{H_3}$$ have $$s{p^3}$$ geometry.
(iv) $$Xe{F_4}$$ has $$s{p^3}{d^2}$$ hybridisation while $$Xe{O_4}$$ has $$s{p^3}$$ hybridisation.
Structure of $$Xe{F_4}$$
.PNG)
Structure of $$Xe{O_4}$$
.PNG)
Hence, $$Xe{F_4}$$ and $$Xe{O_4}$$ are not isostructural.