Question
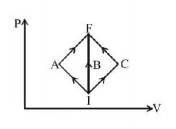
In the $$P-V$$ diagram, $$I$$ is the initial state and $$F$$ is the final state. The gas goes from $$I$$ to $$F$$ by (i) $$IAF,$$ (ii) $$IBE,$$ (iii) $$ICF.$$ The heat absorbed by the gas is
In the $$P-V$$ diagram, $$I$$ is the initial state and $$F$$ is the final state. The gas goes from $$I$$ to $$F$$ by (i) $$IAF,$$ (ii) $$IBE,$$ (iii) $$ICF.$$ The heat absorbed by the gas is
A.
the same in all three processes
B.
the same in (i) and (ii)
C.
greater in (i) than in (ii)
D.
the same in (i) and (iii)
Answer :
greater in (i) than in (ii)
Solution :
Heat absorbed in a thermodynamic process is given by $$\Delta Q = \Delta U + \Delta W.$$
Here $$\Delta U$$ is same for all the three processes as it depends only on initial and final states.
$$\eqalign{ & {\text{But}}\,\Delta {W_I} = + Ve,\Delta {W_{II}} = 0,\Delta {W_{III}} = - ve \cr & \therefore \Delta {Q_I} > \Delta {Q_{II}} \cr} $$
Heat absorbed in a thermodynamic process is given by $$\Delta Q = \Delta U + \Delta W.$$
Here $$\Delta U$$ is same for all the three processes as it depends only on initial and final states.
$$\eqalign{ & {\text{But}}\,\Delta {W_I} = + Ve,\Delta {W_{II}} = 0,\Delta {W_{III}} = - ve \cr & \therefore \Delta {Q_I} > \Delta {Q_{II}} \cr} $$
