Question
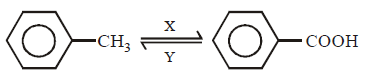
In the above sequence of reaction $$X$$ and $$Y$$ are respectively
A.
\[{{H}_{2}}/Pt;B{{r}_{2}}\]
B.
\[KMn{{O}_{4}};{{H}_{2}}/Pt\]
C.
\[KMn{{O}_{3}}\left( aq \right);HI/P\]
D.
\[N{{H}_{2}}-N{{H}_{2}}/KOH,HI/P\]
Answer :
\[KMn{{O}_{3}}\left( aq \right);HI/P\]
Solution :
\[KMn{{O}_{4}}\] converts \[-C{{H}_{3}}\] group of toluene into \[-COOH\] while $$HI$$ reduces \[-COOH\] group into \[-C{{H}_{3}}\] group.
\[KMn{{O}_{4}}\] converts \[-C{{H}_{3}}\] group of toluene into \[-COOH\] while $$HI$$ reduces \[-COOH\] group into \[-C{{H}_{3}}\] group.