Question
In $$PO_4^{3 - }$$ ion, the formal charge on each oxygen atom and $$P - O$$ bond order respectively are
A.
$$- 0.75, 0.6$$
B.
$$ 0.75, 1.0$$
C.
$$- 0.75, 1.25$$
D.
$$- 3, 1.25$$
Answer :
$$- 0.75, 1.25$$
Solution :
$$\eqalign{ & P - O\,\,{\text{bond order}} \cr & = \frac{{{\text{Total Number of bonds in all possible direction}}\,{\text{between two atoms }}}}{{{\text{Total number of resonating structures }}}} \cr & = \frac{{2 + 1 + 1 + 1}}{4} \cr & = \frac{5}{4} \cr & = 1.25 \cr & \therefore {\text{Bond}}\,{\text{order}} = 1.25 \cr & {\text{Resonating structures are}} \cr} $$
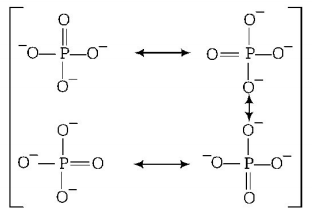
$$\eqalign{ & {\text{Total charge on}}\,\,PO_4^{3 - }\,\,{\text{ion}}\,\,{\text{is}}\,\, - 3 \cr & = \frac{{{\text{Total}}\,\,{\text{charge}}}}{{{\text{Total}}\,\,{\text{entity}}\,\,{\text{of}}\,\,O - {\text{atom}}}} \cr} $$
So, the average formal charge on each $${O - {\text{atom}}}$$ is
$$\eqalign{ & = - \frac{3}{4} \cr & = - 0.75 \cr} $$
$$\eqalign{ & P - O\,\,{\text{bond order}} \cr & = \frac{{{\text{Total Number of bonds in all possible direction}}\,{\text{between two atoms }}}}{{{\text{Total number of resonating structures }}}} \cr & = \frac{{2 + 1 + 1 + 1}}{4} \cr & = \frac{5}{4} \cr & = 1.25 \cr & \therefore {\text{Bond}}\,{\text{order}} = 1.25 \cr & {\text{Resonating structures are}} \cr} $$

$$\eqalign{ & {\text{Total charge on}}\,\,PO_4^{3 - }\,\,{\text{ion}}\,\,{\text{is}}\,\, - 3 \cr & = \frac{{{\text{Total}}\,\,{\text{charge}}}}{{{\text{Total}}\,\,{\text{entity}}\,\,{\text{of}}\,\,O - {\text{atom}}}} \cr} $$
So, the average formal charge on each $${O - {\text{atom}}}$$ is
$$\eqalign{ & = - \frac{3}{4} \cr & = - 0.75 \cr} $$