Question
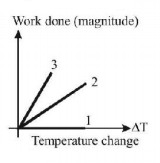
For an ideal gas graph is shown for three processes. Process 1, 2 and 3 are respectively.
For an ideal gas graph is shown for three processes. Process 1, 2 and 3 are respectively.
A.
Isobaric, adiabatic, isochoric
B.
Adiabatic, isobaric, isochoric
C.
Isochoric, adiabatic, isobaric
D.
Isochoric, isobaric, adiabatic
Answer :
Isochoric, isobaric, adiabatic
Solution :
Isochoric proceess $$dV = 0$$
$$W = 0$$ proceess 1
Isobaric : $$W = P\Delta V = nR\Delta T$$
Adiabatic $$\left| W \right| = \frac{{nR\Delta T}}{{\gamma - 1}}$$
$$0 < \gamma - 1 < 1$$
As work done in case of adiabatic process is more so process 3 is adiabatic and process 2 is isobaric.
Isochoric proceess $$dV = 0$$
$$W = 0$$ proceess 1
Isobaric : $$W = P\Delta V = nR\Delta T$$
Adiabatic $$\left| W \right| = \frac{{nR\Delta T}}{{\gamma - 1}}$$
$$0 < \gamma - 1 < 1$$
As work done in case of adiabatic process is more so process 3 is adiabatic and process 2 is isobaric.
