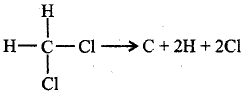251. Enthalpy change for the reaction, $$4H\left( g \right) \to 2{H_2}\left( g \right)$$ is $$ - 869.6\,kJ.$$ The dissociation energy of $$H-H$$ bond is
A
$$ - 869.6\,kJ$$
B
$$ + 434.8\,kJ$$
C
$$ + 217.4\,kJ$$
D
$$ - 434.8\,kJ$$
Answer :
$$ + 434.8\,kJ$$
252.
What will be $$\Delta H$$ for the reaction, $$C{H_2}C{l_2} \to C + 2H + 2Cl?$$
( $$B.E.$$ of $$C- H$$ and $$C- Cl$$ bonds are $$416\,kJ\,mo{l^{ - 1}}$$ and $$325\,kJ\,mo{l^{ - 1}}$$ respectively )
A
832$$\,kJ$$
B
1482$$\,kJ$$
C
650$$\,kJ$$
D
1855$$\,kJ$$
Answer :
1482$$\,kJ$$
253. The heat of combustion of $$C,S$$ and $$C{S_2}$$ are $$ - 393.3\,kJ, - 293.7\,kJ$$ and $$ - 1108.76\,kJ.$$ What will be the heat of formation of $$C{S_2}?$$
A
$$ - 128.06\,kJ$$
B
$$ + 970\,kJ$$
C
$$ + 1108.7\,kJ$$
D
$$ + 12\,kJ$$
Answer :
$$ - 128.06\,kJ$$
254. The enthalpies of elements in their standard states are taken as zero. The enthalpy of formation of a compound
A
is always negative
B
is always positive
C
may be positive or negative
D
is never negative.
Answer :
may be positive or negative
255.
For complete combustion of ethanol,
$${C_2}{H_5}OH\left( \ell \right) + 3{O_2}\left( g \right) \to $$ $$2C{O_2}\left( g \right) + 3{H_2}O\left( \ell \right),$$
the amount of heat produced as measured in bomb calorimeter, is $$1364.47\,kJ\,mo{l^{ - 1}}$$ at $${25^ \circ }C.$$ Assuming ideality the enthalpy of combustion, $${\Delta _c}H,$$ for the reaction will be :
$$\left( {R = 8.314\,kJ\,mo{l^{ - 1}}} \right)$$
A
$$ - 1366.95\,kJ\,mo{l^{ - 1}}$$
B
$$ - 1361.95\,kJ\,mo{l^{ - 1}}$$
C
$$ - 1460.95\,kJ\,mo{l^{ - 1}}$$
D
$$ - 1350.50\,kJ\,mo{l^{ - 1}}$$
Answer :
$$ - 1366.95\,kJ\,mo{l^{ - 1}}$$
256.
At $${25^ \circ }C,$$ when $$1\,mole$$ of $$MgS{O_4}$$ was dissolved in water, the heat evolved was found to be $$91.2\,kJ.$$ One $$mole$$ of $$MgS{O_4}.$$ $$7{H_2}O$$ on dissolution gives a solution of the same composition accompanied by an absorption of $$13.8\,kJ.$$ The enthalpy of hydration, i.e., $$\Delta {H_h}$$ for the reaction
$$MgS{O_4}\left( s \right) + 7{H_2}O\left( l \right) \to MgS{O_4}.7{H_2}O\left( s \right)$$ is :
A
$$ - 105\,kJ/mol$$
B
$$ - 77.4\,kJ/mol$$
C
$$105\,kJ/mol$$
D
$${\text{None of these}}$$
Answer :
$$ - 105\,kJ/mol$$
257. System in which there is no exchange of matter, work or energy from surroundings is
A
closed
B
adiabatic
C
isolated
D
isothermal
Answer :
isolated
258. For a reaction to be spontaneous at any temperature, the conditions are
A
$$\Delta H = + ve,\Delta S = + ve$$
B
$$\Delta H = - ve,\Delta S = - ve$$
C
$$\Delta H = + ve,\Delta S = - ve$$
D
$$\Delta H = - ve,\Delta S = + ve$$
Answer :
$$\Delta H = - ve,\Delta S = + ve$$
259. The heat of combustion of carbon to $$C{O_2}$$ is $$ - 393.5kJ/mol.$$ The heat released upon the formation of $$35.2\,g$$ of $$C{O_2}$$ from carbon and oxygen gas is
A
$$ - 315\,kJ$$
B
$$ + 315\,kJ$$
C
$$ - 630\,kJ$$
D
$$ - 3.15\,kJ$$
Answer :
$$ - 315\,kJ$$
260.
Assume each reaction is carried out in an open container. For which reaction will $$\Delta H = \Delta E?$$
A
$${H_2}\left( g \right) + B{r_2}\left( g \right) \to 2HBr\left( g \right)$$
B
$$C\left( s \right) + 2{H_2}O\left( g \right) \to 2{H_2}\left( g \right) + C{O_2}\left( g \right)$$
C
$$PC{l_5}\left( g \right) \to PC{l_3}\left( g \right) + C{l_2}\left( g \right)$$
D
$$2CO\left( g \right) + {O_2}\left( g \right) \to 2\,C{O_2}\left( g \right)$$
Answer :
$${H_2}\left( g \right) + B{r_2}\left( g \right) \to 2HBr\left( g \right)$$