71. The carbonyl compound producing an optically active product by reaction with $$LiAl{H_4}$$ is
A
propanone
B
butanone
C
3-pentanone
D
benzophenone
Answer :
butanone
72. The enolic form of acetone contains
A
9 sigma bonds, 1 pi - bond and 2 lone pairs
B
8 sigma bonds, 2 pi - bonds and 2 lone pairs
C
10 sigma bonds, 1 pi-bond and 1 lone pair
D
9 sigma bonds, 2 pi - bonds and 1 lone pair
Answer :
9 sigma bonds, 1 pi - bond and 2 lone pairs
73. $$m{\text{ - }}$$ Chlorobenzaldehyde on reaction with conc. $$KOH$$ at room temperature gives
A
potassium $$m{\text{ - }}$$chlorobenzoate and
$$m{\text{ - }}$$hydroxybenzaldehyde
B
$$m{\text{ - }}$$hydroxybenzaldehyde and $$m{\text{ - }}$$chlorobenzyl alcohol
C
$$m{\text{ - }}$$chlorobenzyl alcohol and $$m{\text{ - }}$$hydroxybenzyl alcohol
D
potassium $$m{\text{ - }}$$chlorobenzoate and $$m{\text{ - }}$$chlorobenzyl
alcohol.
Answer :
potassium $$m{\text{ - }}$$chlorobenzoate and $$m{\text{ - }}$$chlorobenzyl
alcohol.
74. 1-phenyl ethanol can be prepared by the reaction of benzaldehyde with
A
methyl bromide
B
ethyl iodide and magnesium
C
methyl iodide and magnesium
D
methyl bromide and aluminium bromide
Answer :
methyl iodide and magnesium
75.
Study the following sequence of reactions and identify the compounds $$(X)$$ and $$(Y).$$

$$X$$
$$Y$$
(a)
Benzaldehyde
Benzoic acid
(b)
2-Chlorotoluene
2-Chlorobenzoic acid
(c)
2, 4-Dichlorobenzene
Benzoic acid
(d)
Benzal chloride
Benzaldehyde
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(d)
76.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
Ethanal, Propanal, Propanone, Butanone
A
Butanone < Propanone < Propanal < Ethanal
B
Propanone < Butanone < Ethanal > Propanal
C
Propanal < Ethanal < Propanone < Butanone
D
Ethanal < Propanal < Propanone < Butanone
Answer :
Butanone < Propanone < Propanal < Ethanal
77. Addition of water to alkynes occurs in acidic medium and in the presence of $$H{g^{2 + }}$$ ions as a catalyst. Which of the following products will be formed on addition of water to but-1-yne under these conditions?
A
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,H\]
B
\[C{{H}_{3}}-C{{H}_{2}}\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,C{{H}_{3}}\]
C
\[C{{H}_{3}}-C{{H}_{2}}\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,OH+C{{O}_{2}}\]
D
\[C{{H}_{3}}\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,OH+H\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,H\]
Answer :
\[C{{H}_{3}}-C{{H}_{2}}\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,C{{H}_{3}}\]
78.
The reagents employed to carry the following transformation

A
\[LiAl{{H}_{4}},{{H}_{2}}S{{O}_{4}}/\text{heat}\]
B
\[PCC/C{{H}_{2}}C{{l}_{2}}\,\text{followed by}\,HI{{O}_{4}}\]
C
\[NaB{{H}_{4}}/C{{H}_{3}}OH\,\text{followed by}\,HI{{O}_{4}}\]
D
\[{{O}_{3}}\,\text{followed by}\,{{\left( C{{H}_{3}} \right)}_{2}}S\]
Answer :
\[NaB{{H}_{4}}/C{{H}_{3}}OH\,\text{followed by}\,HI{{O}_{4}}\]
79. lodoform test is not given by
A
2-pentanone
B
ethanol
C
ethanal
D
3-pentanone
Answer :
3-pentanone
80.
Correct order of reactivity of following compounds towards Grignard reagent ?

A
I > II > III
B
II > I > III
C
II > III > I
D
I > III > II
Answer :
II > I > III
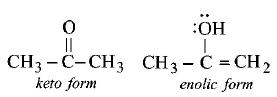




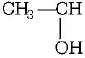 group give positive
iodoform test. In 2-pentanone, $$\left( {C{H_3}C{H_2}C{H_2}COC{H_3}} \right),C{H_3}CHO$$ and $${C_2}{H_5}OH,$$ required groups are present, thus they give iodoform as follows
group give positive
iodoform test. In 2-pentanone, $$\left( {C{H_3}C{H_2}C{H_2}COC{H_3}} \right),C{H_3}CHO$$ and $${C_2}{H_5}OH,$$ required groups are present, thus they give iodoform as follows