Question
An organic compound having molecular mass 60 is found to contain $$C = 20\% ,H = 6.67\% \,{\text{and}}\,N = 46.67\% $$ while rest is oxygen. On heating it gives $$N{H_3}$$ alongwith a solid residue. The solid residue give violet colour with alkaline copper sulphate solution. The compound is
A.
$$C{H_3}C{H_2}CON{H_2}$$
B.
$${\left( {N{H_2}} \right)_2}CO$$
C.
$$C{H_3}CON{H_2}$$
D.
$$C{H_3}NCO$$
Answer :
$${\left( {N{H_2}} \right)_2}CO$$
Solution :
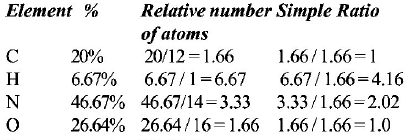
The compound is $$C{H_4}{N_2}O$$
$${\text{Empirical weight}} = 60;\,Mol.\,wt. = 60;\,\,\,\therefore \,\,n = \frac{{60}}{{60}} = 1$$
$${\text{Molecular formula}} = C{H_4}{N_2}O;$$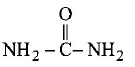
On heating urea loses ammonia to give Biuret
$$2N{H_2}CON{H_2} \to {H_2}NCO.NH.CON{H_2} + N{H_3}$$
Biuret with alkaline $$CuS{O_4}$$ gives violet colour. Test for $$ - CONH - $$ group.
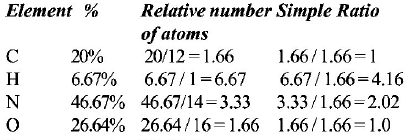
The compound is $$C{H_4}{N_2}O$$
$${\text{Empirical weight}} = 60;\,Mol.\,wt. = 60;\,\,\,\therefore \,\,n = \frac{{60}}{{60}} = 1$$
$${\text{Molecular formula}} = C{H_4}{N_2}O;$$
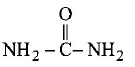
On heating urea loses ammonia to give Biuret
$$2N{H_2}CON{H_2} \to {H_2}NCO.NH.CON{H_2} + N{H_3}$$
Biuret with alkaline $$CuS{O_4}$$ gives violet colour. Test for $$ - CONH - $$ group.