Question
Among the following compound one that is most reactive towards electrophilic nitration is
A.
benzoic acid
B.
nitrobenzene
C.
toluene
D.
benzene
Answer :
toluene
Solution :
Presence of electron releasing groups like $$-R,-OH,$$ etc. increases the electron density at $$o/p$$ - position and thus, makes the benzene ring more reactive ( at $$o/p$$ - positions ) towards an electrophile. On the other hand, electron withdrawing groups like $$ - COOH, - N{O_2},$$ etc. if present, reduces electron density and thus, reduces the activity of benzene nucleus towards an electrophile. Thus, the order of the given compounds towards electrophilic nitration is
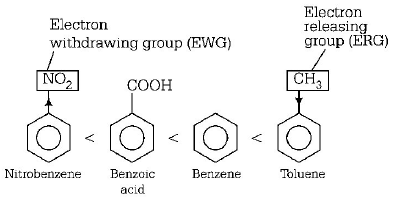
Thus, toluene is most reactive towards electrophilic nitration.
Presence of electron releasing groups like $$-R,-OH,$$ etc. increases the electron density at $$o/p$$ - position and thus, makes the benzene ring more reactive ( at $$o/p$$ - positions ) towards an electrophile. On the other hand, electron withdrawing groups like $$ - COOH, - N{O_2},$$ etc. if present, reduces electron density and thus, reduces the activity of benzene nucleus towards an electrophile. Thus, the order of the given compounds towards electrophilic nitration is

Thus, toluene is most reactive towards electrophilic nitration.