Question
A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half. Then
A.
compressing the gas through adiabatic process will require more work to be done.
B.
compressing the gas isothermally or adiabatically will require the same amount of work.
C.
which of the case (whether compression through isothermal or through adiabatic process) requires more work will depend upon the atomicity of the gas.
D.
compressing the gas isothermally will require more work to be done.
Answer :
compressing the gas through adiabatic process will require more work to be done.
Solution :
The solution of this question can be understood by plotting a $$p-V$$ graph for the compression of a gas isothermally and adiabatically simultaneously to half of its initial volume. i.e.
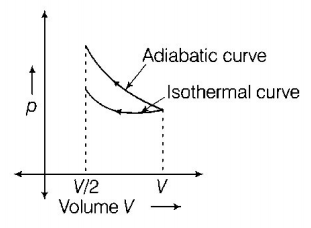
Since, the isothermal curve is less steeper than the adiabatic curve. So, area under the $$p-V$$ curve for adiabatic process has more magnitude than isothermal curve. Hence, work done in adiabatic process will be more than in isothermal process.
The solution of this question can be understood by plotting a $$p-V$$ graph for the compression of a gas isothermally and adiabatically simultaneously to half of its initial volume. i.e.

Since, the isothermal curve is less steeper than the adiabatic curve. So, area under the $$p-V$$ curve for adiabatic process has more magnitude than isothermal curve. Hence, work done in adiabatic process will be more than in isothermal process.
