Question
A dichloroderivative $$(A)$$ on treating with $$KCN$$ followed by acid hydrolysis and heating gives a monobasic acid $$(B)$$ along with liberation of $$C{O_2}.$$ $$(B)$$ on heating with liquid ammonia followed by treating with $$B{r_2}/KOH$$ gives $$(C)$$ which on treating with $$NaN{O_2}$$ and $$HCl$$ at low temperature followed by oxidation gives a monobasic acid $$(D)$$ having molecular mass 74. $$'C'$$ in the whole process would be
A.
ethyl amine
B.
propyl amine
C.
$$tert$$ - butyl amine
D.
cyclopentyl amine
Answer :
propyl amine
Solution :
The overall reaction can be summarized as follows:
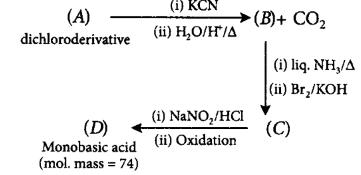
Structure of $$(D)$$ can be :
$$RCOOH = 74,$$
$$\eqalign{ & R = 74 - \left( {COOH} \right) \cr & \,\,\,\,\,\, = 74 - 45 \cr & \,\,\,\,\,\, = 29 \cr} $$
i.e., $$R = {C_2}{H_5}$$ and hence acid $$D$$ is $$C{H_3} - C{H_2} - COOH.$$
Now $$(C)$$ will be $$C{H_3} - C{H_2} - C{H_2}N{H_2}$$ which under given conditions gives $$(D)$$ and thus, $$(B)$$ will be butanoic acid which first forms amide. This amide undergoes Hoffmann bromamide reaction to give $$n$$ - propylamine $$(C).$$ To get butanoic acid $$(B)$$ under given conditions dihalide $$(A)$$ will be 1, 1-dichloropropane only. The reactions can be represented as :

The overall reaction can be summarized as follows:

Structure of $$(D)$$ can be :
$$RCOOH = 74,$$
$$\eqalign{ & R = 74 - \left( {COOH} \right) \cr & \,\,\,\,\,\, = 74 - 45 \cr & \,\,\,\,\,\, = 29 \cr} $$
i.e., $$R = {C_2}{H_5}$$ and hence acid $$D$$ is $$C{H_3} - C{H_2} - COOH.$$
Now $$(C)$$ will be $$C{H_3} - C{H_2} - C{H_2}N{H_2}$$ which under given conditions gives $$(D)$$ and thus, $$(B)$$ will be butanoic acid which first forms amide. This amide undergoes Hoffmann bromamide reaction to give $$n$$ - propylamine $$(C).$$ To get butanoic acid $$(B)$$ under given conditions dihalide $$(A)$$ will be 1, 1-dichloropropane only. The reactions can be represented as :
